CONTRAINDICATIONS
ONIVYDE is contraindicated in patients who have experienced a severe hypersensitivity reaction or anaphylaxis to ONIVYDE or irinotecan HCl.
WARNINGS AND PRECAUTIONS
Severe Neutropenia: ONIVYDE can cause severe or life-threatening neutropenia and fatal neutropenic sepsis. In NAPOLI 3, Grade 3 and 4 neutropenia occurred in 26% of patients receiving ONIVYDE in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) and fatal neutropenic fever in 0.3% of patients. In NAPOLI 3, the incidence of Grade 3 or 4 neutropenia was similar among Asian patients [6 of 20 (30%)] compared to White patients [76 of 289 (26%)]. Neutropenic fever/neutropenic sepsis was reported in 5% of Asian patients (1 of 20) compared to 2.3% of White patients (7 of 306). In NAPOLI-1, Grade 3 and 4 neutropenia occurred in 20% of patients receiving ONIVYDE in combination with fluorouracil and leucovorin (ONIVYDE/FU/LV). Neutropenic sepsis occurred in 3% and fatal neutropenic sepsis in 0.8%. In NAPOLI-1, the incidence of Grade 3 or 4 neutropenia was higher among Asian patients [18 of 33 (55%)] compared to White patients [13 of 73 (18%)]. Neutropenic fever/neutropenic sepsis was reported in 6% of Asian patients compared to 1% of White patients.
Monitor complete blood cell counts on Days 1 and 8 of every cycle and more frequently if clinically indicated. Withhold ONIVYDE if the absolute neutrophil count (ANC) is below 1500/mm3 or if neutropenic fever occurs. Resume ONIVYDE when the ANC is 1500/mm3 or above. Reduce ONIVYDE dose for Grade 3-4 neutropenia or neutropenic fever following recovery in subsequent cycles.
Severe Diarrhea: In NAPOLI 3, Grade 3 and 4 diarrhea (early-onset [within 24 hours of chemotherapy] and late-onset [more than
24 hours following chemotherapy]) occurred in 20% receiving NALIRIFOX. In NAPOLI-1, Grade 3 or 4 diarrhea occurred in 13% receiving ONIVYDE/FU/LV. The incidence of Grade 3 or 4 late-onset diarrhea was 9% in patients receiving ONIVYDE/FU/LV. The incidence of Grade 3 or 4 early-onset diarrhea was 3% in patients receiving ONIVYDE/FU/LV.
To reduce the risk of severe diarrhea, patients should stop lactose-containing products, eat a low-fat diet, and maintain hydration during treatment with ONIVYDE. Withhold ONIVYDE for Grade 2-4 diarrhea. Local institutional guidelines should be followed for the treatment of diarrhea that does not improve within 48 hours and may include the addition of diphenoxylate hydrochloride plus atropine sulfate or octreotide. Following recovery to Grade 1 diarrhea, resume ONIVYDE at a reduced dose.
Interstitial Lung Disease (ILD): ONIVYDE can cause severe and fatal ILD. Postmarketing cases of severe and fatal ILD have been reported with ONIVYDE. Risk factors include pre-existing lung disease, use of pneumotoxic medicinal products, colony stimulating factors or having previously received radiation therapy. Patients with risk factors should be closely monitored for respiratory symptoms before and during ONIVYDE therapy. Withhold ONIVYDE in patients with new or progressive dyspnea, cough, and fever, pending diagnostic evaluation. Discontinue ONIVYDE in patients with a confirmed diagnosis of ILD.
Severe Hypersensitivity Reaction: Irinotecan, including ONIVYDE, can cause severe hypersensitivity reactions, including anaphylactic reactions. Permanently discontinue ONIVYDE in patients who experience a severe hypersensitivity reaction.
Embryo-Fetal Toxicity: Based on animal data with irinotecan HCl and the mechanism of action of ONIVYDE, ONIVYDE can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during and for 7 months after the last dose of ONIVYDE treatment.
ADVERSE REACTIONS FOR NALIRIFOX
- The most common adverse reactions (≥20%) of NALIRIFOX were diarrhea (72%), fatigue (62%), nausea (59%), vomiting (40%), decreased appetite (37%), abdominal pain (35%), mucosal inflammation (28%), constipation (25%), and weight decreased (22%).
- Permanent discontinuation of ONIVYDE due to an adverse reaction occurred in 17% of patients. Adverse reactions that resulted in permanent discontinuation of ONIVYDE in ≥1% of patients included neutropenia, thrombocytopenia, diarrhea, fatigue, infections, and cerebrovascular accident.
- Dosage reduction of ONIVYDE due to an adverse reaction occurred in 52% of patients. Adverse reactions that required dosage reduction in ≥1% of patients included anemia, decreased appetite, diarrhea, fatigue, febrile neutropenia, hypokalemia, liver function test abnormalities, nausea, mucosal inflammation, neutropenia, peripheral neuropathy, vomiting, thrombocytopenia, and weight decreased.
- Dosage interruptions of ONIVYDE due to an adverse reaction occurred in 1.9% of patients. Adverse reactions which required dosage interruption in ≥0.5% of patients included hypersensitivity and infusion-related reaction.
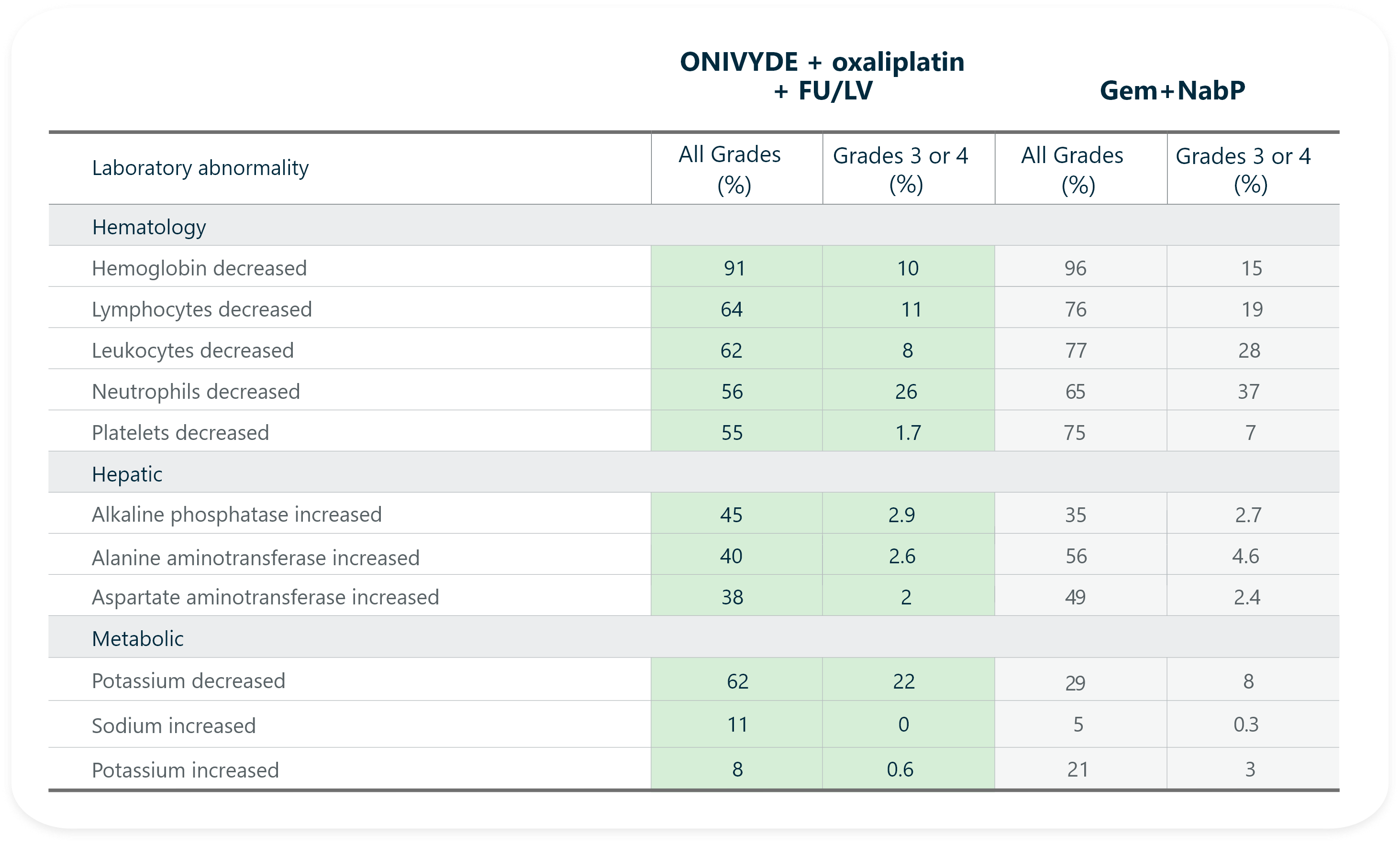
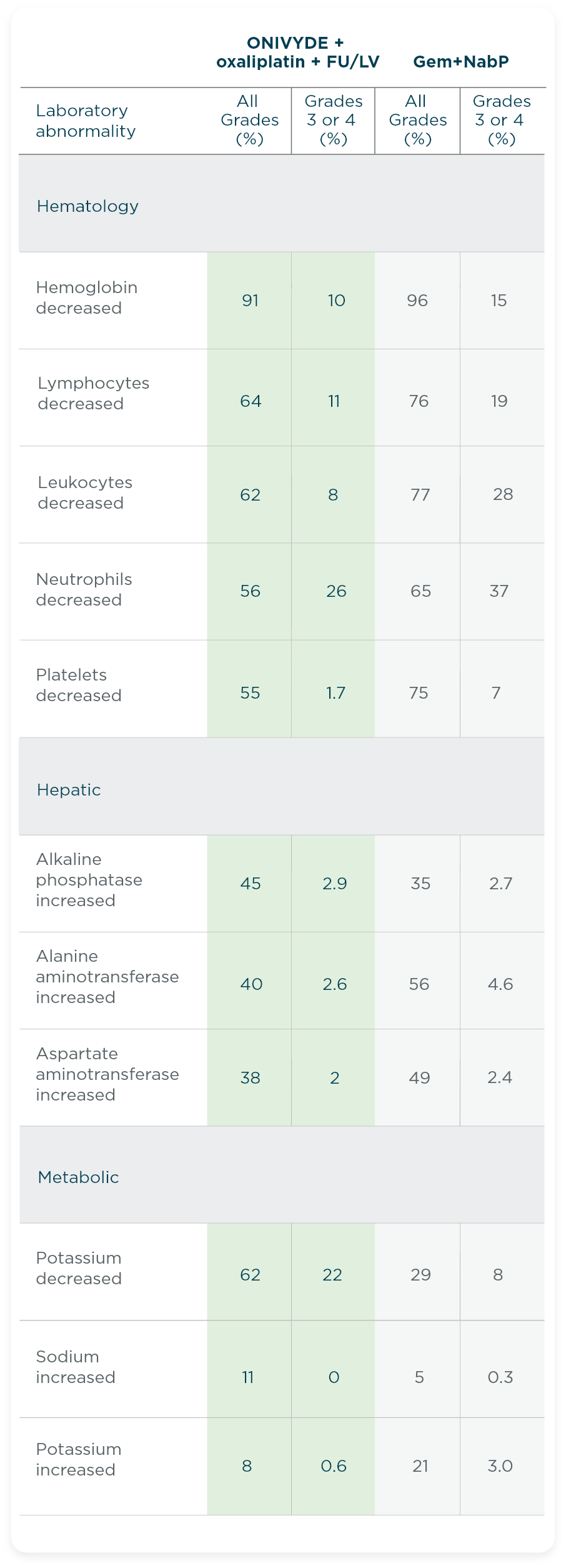
- The most common laboratory abnormalities (≥10% Grade 3 or 4) were decreased neutrophils (26%), decreased potassium (22%), decreased lymphocytes (11%), and decreased hemoglobin (10%).
ADVERSE REACTIONS FOR ONIVYDE/FU/LV
- The most common adverse reactions (≥20%) were diarrhea (59%), fatigue/asthenia (56%), vomiting (52%), nausea (51%), decreased appetite (44%), stomatitis (32%), and pyrexia (23%).
- Adverse reactions led to permanent discontinuation of ONIVYDE in 11% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions resulting in discontinuation of ONIVYDE were diarrhea, vomiting, and sepsis.
- Dose reductions of ONIVYDE for adverse reactions occurred in 33% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions requiring dose reductions were neutropenia, diarrhea, nausea, and anemia.
- ONIVYDE was withheld or delayed for adverse reactions in 62% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions requiring interruption or delays were neutropenia, diarrhea, fatigue, vomiting, and thrombocytopenia.
- The most common severe laboratory abnormalities (≥10% Grade 3 or 4) were lymphopenia and neutropenia.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of ONIVYDE:
- Hypersensitivity (including anaphylactic reaction and angioedema).
DRUG INTERACTIONS
- Avoid the use of strong CYP3A4 inducers, if possible, and substitute non-enzyme inducing therapies ≥2 weeks prior to initiation of ONIVYDE.
- Avoid the use of strong CYP3A4 or UGT1A1 inhibitors, if possible, and discontinue strong CYP3A4 inhibitors ≥1 week prior to starting therapy.
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data with irinotecan HCl and the mechanism of action of ONIVYDE, ONIVYDE can cause fetal harm when administered to a pregnant woman. There are no available data in pregnant women. Advise pregnant women of the potential risk to a fetus.
- Lactation: Advise nursing women not to breastfeed during and for 1 month after the last dose of ONIVYDE.
To report SUSPECTED ADVERSE REACTIONS, contact Ipsen Biopharmaceuticals, Inc. at 1-855-463-5127 or FDA at
1-800-FDA-1088 or www.fda.gov/medwatch.
References: 1. ONIVYDE® [package insert]. Cambridge, MA. Ipsen Biopharmaceuticals, Inc.; 2024. 2. Wainberg ZA, Melisi D, Macarulla T, et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial. Lancet. 2023;402(10409):1272-1281. 3. Zhang H. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016;9:3001-3007. 4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Pancreatic Adenocarcinoma V2.2025. ©National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed December 4, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. 5. Dimou A, Syrigos KN, Saif MW. Overcoming the stromal barrier: technologies to optimize drug delivery in pancreatic cancer. Ther Adv Med Oncol. 2012;4(5):271-279. 6. Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66(6):3271-3277. 7. CAMPTOSAR® [package insert]. New York, NY. Pfizer, Inc.; 2022. 8. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. 9. Hsueh C-T, Selim JH, Tsai JY, Hsueh C-T. Nanovectors for anti-cancer drug delivery in the treatment of advanced pancreatic adenocarcinoma. World J Gastroenterol. 2016;22(31):7080-7090. 10. Kelly C, Jefferies C, Cryan S-A. Targeted liposomal drug delivery to monocytes and macrophages. J Drug Deliv. 2011;2011:727241. 11. National Cancer Institute. Drugs approved for pancreatic cancer. https://www.cancer.gov/about-cancer/treatment/drugs/pancreatic. Accessed June 27, 2024. 12. Data on file. Cambridge, MA. Ipsen Biopharmaceuticals, Inc. 2023. 13. Conroy T, Desseigne F, Ychou M, et al; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825. 14. Ipsen data on file: ASCO advisory board roundtable. 2023. 15. Wang-Gillam A, Li C-P, Bodoky G, et al; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545-557. 16. Data on file #1. Basking Ridge, NJ. Ipsen Biopharmaceuticals, Inc.; 2015. 17. Wang-Gillam A, Hubner RA, Siveke JT, et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: final overall survival analysis and characteristics of long-term survivors. Eur J Cancer. 2019;108:78-87. 18. Ipsen data on file: IQVIA medical claims post-gemcitabine usage analysis, Q4 2017–Q1 2023. 19. Department of Health and Human Services. U.S. Food and Drug Administration. ONIVYDE (irinotecan liposome injection) Approval Letter. NDA 207793. October 22, 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207793Orig1s000Approv.pdf. Accessed June 27, 2024. 20. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10-27. doi:10.14740/wjon1166.