ONIVYDE HELPS DELIVER IRINOTECAN TO mPDAC TUMORS1,3,5,6
ONIVYDE is designed differently from irinotecan. Its pegylated, liposomal formulation protects irinotecan from being rapidly cleared from the body1,7,8*
Polyethylene glycol (PEG)
helps prevent the ONIVYDE
liposome from being rapidly
cleared from the body, so
it can stay in circulation longer8
Liposomal
encapsulation
helps protect irinotecan from being converted to its active metabolite (SN-38) before it can reach the tumor site1,3
In a pre-clinical study in equivalent doses, ONIVYDE showed higher and sustained intratumoral levels of irinotecan and SN-38 relative to free irinotecan1,6
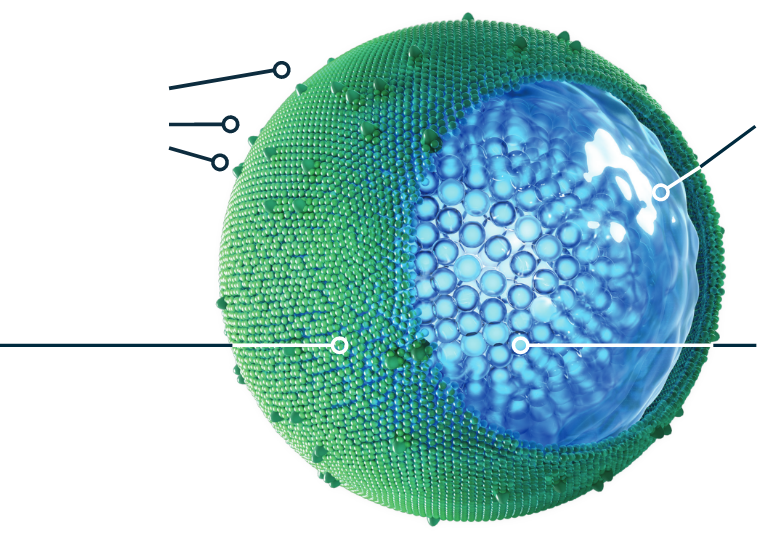
Aqueous space
surrounds the irinotecan
molecules1
Irinotecan molecules
~80,0009
*The clinical significance of this is unknown.
ONIVYDE HELPS DELIVER IRINOTECAN TO mPDAC TUMORS1,3,5,6
ONIVYDE is designed differently from irinotecan. Its pegylated, liposomal formulation protects irinotecan from being rapidly cleared from the body1,7,8*
Polyethylene glycol (PEG)
helps prevent the ONIVYDE liposome from being rapidly cleared from the body, so it can stay in circulation longer8
Liposomal
encapsulation
helps protect irinotecan from being converted to its active metabolite (SN-38) before it can reach the tumor site1,3
In a pre-clinical study in equivalent doses, ONIVYDE showed higher and sustained intratumoral levels of irinotecan and SN-38 relative to free irinotecan1,6
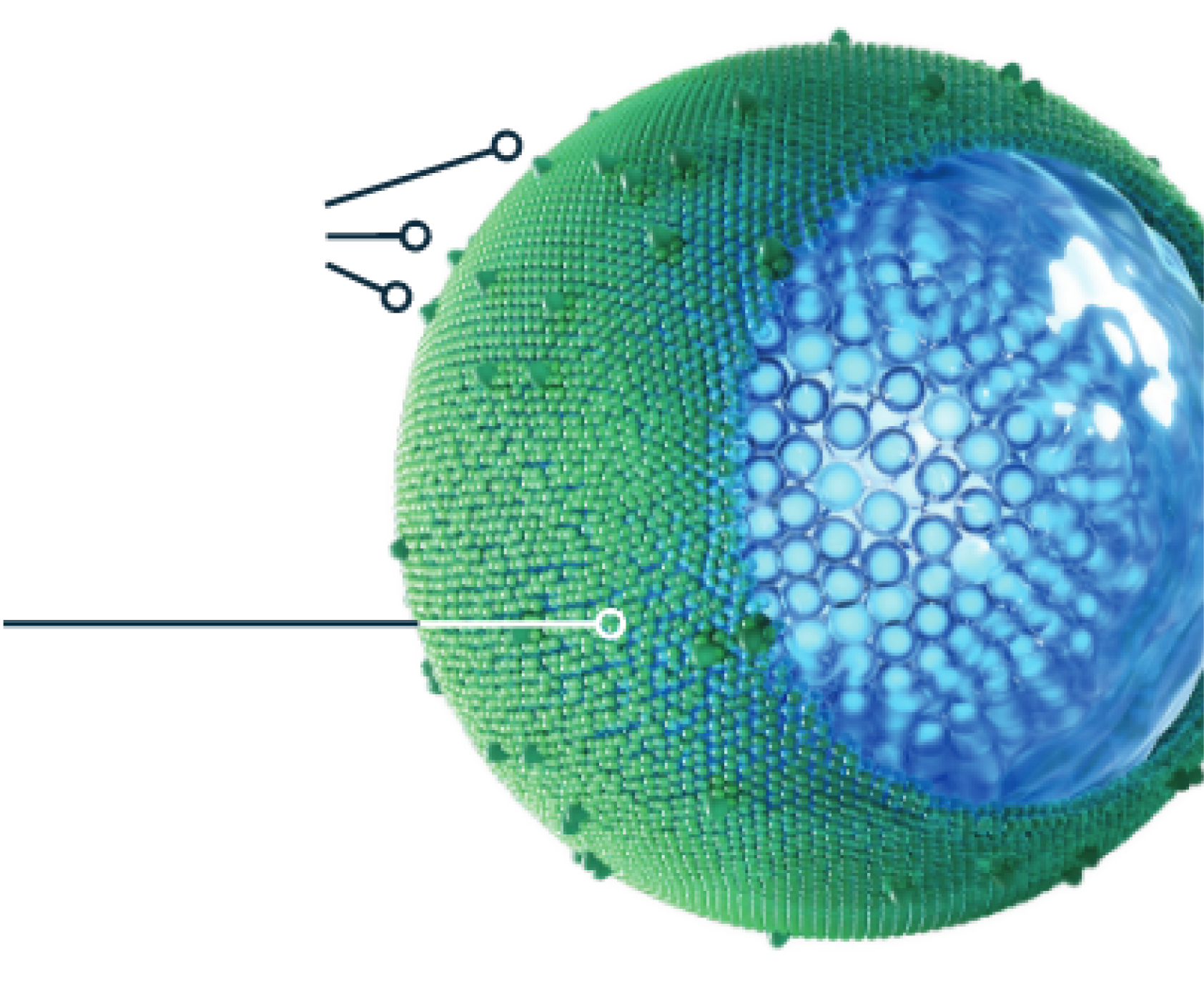
Aqueous space
surrounds the irinotecan molecules1
Irinotecan molecules
~80,0009
*The clinical significance of this is unknown.
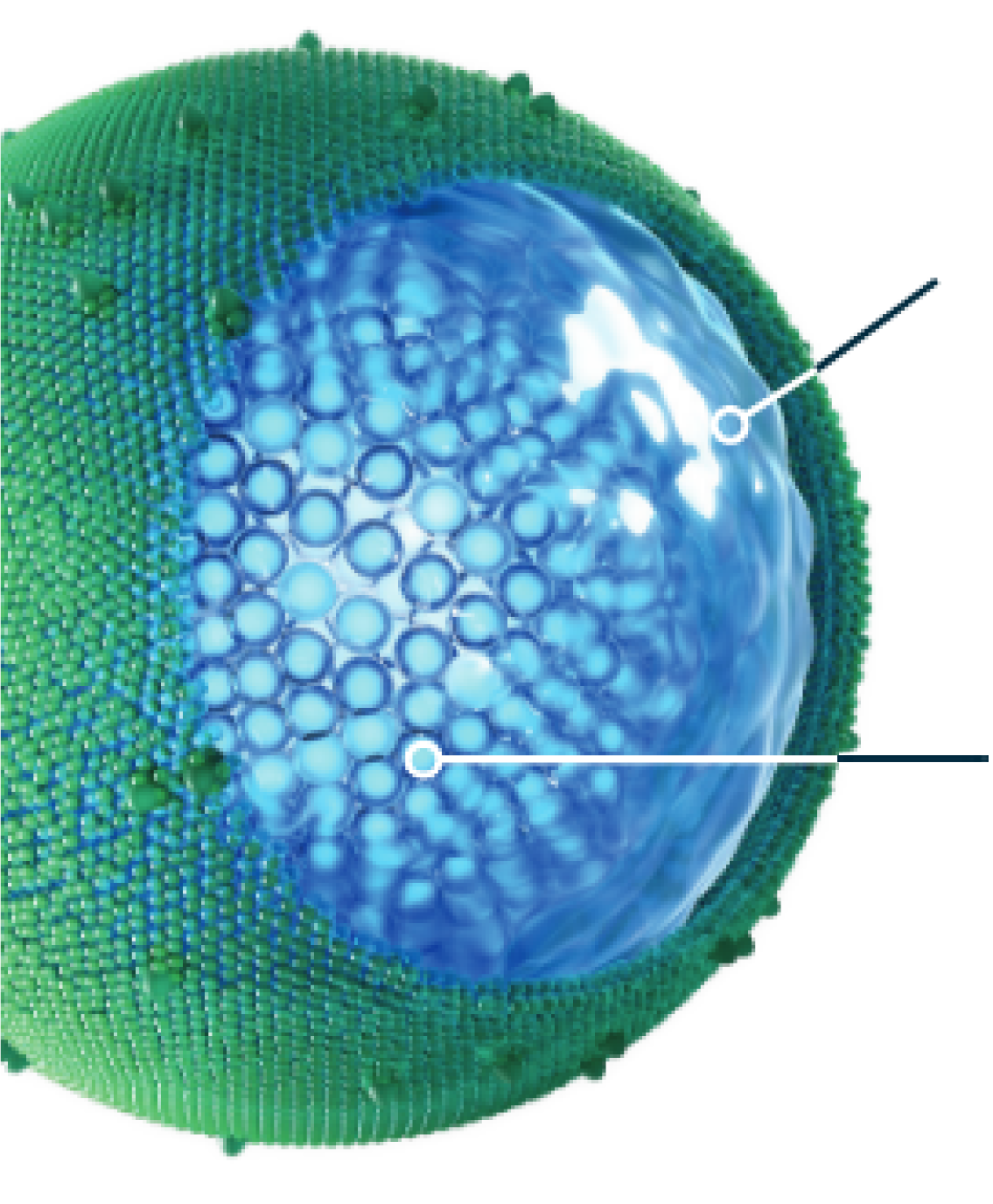
Protection of irinotecan and prolonged circulation time allow
ONIVYDE to deliver its cytotoxic payload to the tumor3
ONIVYDE was designed to address the challenge of the dense stroma in pancreatic tumors1,5
Prolonged circulation
- 95% of irinotecan remained liposomal encapsulated in circulation1
- The ratios between total and encapsulated forms did not change from 0 to 170 hours post-dose1
- The half-life of liposomal irinotecan in the body is 1.93 days for the 50 mg/m2 dose and 1.87 days for the 70 mg/m2 dose1

mPDAC tumor access
- Tumors often have leaky vasculature and defective lymphatic drainage, which allow larger particles—such as macromolecules and liposomes—to gain access and be retained3,5
- This is believed to enable the ONIVYDE liposome to exit circulation and reach the tumor3,5
Intratumoral activity
- Tumor-associated macrophages engulf and unpack the ONIVYDE liposomes, releasing irinotecan and converting it to SN-38, its more potent active metabolite6,10
ONIVYDE mode of delivery video
Watch how the ONIVYDE liposome addresses the challenges of the dense stroma surrounding mPDAC tumors.
INDICATIONS
- ONIVYDE® (irinotecan liposome injection) is indicated, in combination with oxaliplatin, fluorouracil, and leucovorin, for the first-line treatment of adult patients with metastatic pancreatic adenocarcinoma.
- ONIVYDE is indicated, in combination with fluorouracil and leucovorin, for the treatment of adult patients with metastatic pancreatic adenocarcinoma after disease progression following gemcitabine-based therapy.
Limitations of Use: ONIVYDE is not indicated as a single agent for the treatment of patients with metastatic pancreatic adenocarcinoma.
IMPORTANT SAFETY INFORMATION
WARNING: SEVERE NEUTROPENIA AND SEVERE DIARRHEA
Neutropenia
- Severe and life-threatening neutropenia, including fatal neutropenic sepsis and fatal neutropenic fever, has occurred in patients receiving ONIVYDE in combination with oxaliplatin, fluorouracil, and leucovorin and in combination with fluorouracil and leucovorin. Withhold ONIVYDE for absolute neutrophil count below 1500/mm3 or neutropenic fever. Monitor blood cell counts periodically during treatment.
Diarrhea
- Severe and life-threatening diarrhea has occurred in patients receiving ONIVYDE in combination with oxaliplatin, fluorouracil, and leucovorin and in combination with fluorouracil and leucovorin. Do not administer ONIVYDE to patients with bowel obstruction. Withhold ONIVYDE for diarrhea of Grade 2-4 severity. Administer loperamide for late diarrhea of any severity. Administer atropine, if not contraindicated, for early diarrhea of any severity.
Please stay tuned for additional Important Safety Information at the end of this video, and please see full Prescribing Information, including BOXED WARNING, at ONIVYDE.com/USPI.
Metastatic pancreatic cancer is considered to be one of the most challenging types of cancer to treat.
The tumors have dense stroma, which can hinder the delivery of some therapeutic agents.
ONIVYDE represents a technological advance in drug delivery. It is a liposomal formulation of irinotecan that was designed to address the challenge of the dense stroma.
This design is different from conventional irinotecan and helps ONIVYDE stay in the fight.
The ONIVYDE liposome is a lipid bilayer vesicle that shelters an aqueous space containing approximately 80,000 irinotecan molecules. This liposomal encapsulation helps protect irinotecan from early conversion to a more active metabolite, SN-38.
The ONIVYDE liposome is stabilized through the addition of a hydrophilic polymer coating, polyethylene glycol, or PEG.
PEGylation helps protect the ONIVYDE liposome from being rapidly cleared from the body, so it can stay in circulation longer.
Following administration of ONIVYDE by intravenous infusion, the half-life of total irinotecan in the body is 1.93 days for the 50 mg/m2 dose and 1.87 days for the 70 mg/m2 dose.
In addition, direct measurement of the ONIVYDE liposome showed that 95% of irinotecan remained liposome-encapsulated in circulation and the ratio between total and encapsulated forms did not change from 0 to 170 hours postdose.
Protection of irinotecan and prolonged circulation time allow ONIVYDE to deliver its cytotoxic payload to the tumor.
Tumors often have leaky vasculature and defective lymphatic drainage allowing larger particles, such as macromolecules and liposomes, to gain access and be retained.
This effect is known as the enhanced permeability and retention—or EPR effect. The EPR effect is believed to enable the ONIVYDE liposome to exit circulation and reach the tumor.
The tumor environment contains a high concentration of macrophages.
These tumor-associated macrophages engulf the ONIVYDE liposomes through phagocytosis and unpack the liposomes, facilitating the release of irinotecan.
The irinotecan is then converted by carboxylesterases into SN-38, its active metabolite.
Both irinotecan and SN-38 are topoisomerase inhibitors, which work by overwhelming the DNA repair mechanisms in cells.
However, SN-38 is more potent than irinotecan.
In a preclinical study in equivalent doses, ONIVYDE showed higher and sustained intratumoral levels of irinotecan and SN-38 relative to conventional irinotecan.
ONIVYDE is a liposomal formulation of irinotecan specifically designed to protect its payload while in circulation, so it can reach the pancreatic tumor and help stay in the fight.
CONTRAINDICATIONS
ONIVYDE is contraindicated in patients who have experienced a severe hypersensitivity reaction or anaphylaxis to ONIVYDE or irinotecan HCl.
WARNINGS AND PRECAUTIONS
Severe Neutropenia: ONIVYDE can cause severe or life-threatening neutropenia and fatal neutropenic sepsis. In NAPOLI 3, Grade 3 and 4 neutropenia occurred in 26% of patients receiving ONIVYDE in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) and fatal neutropenic fever in 0.3% of patients. In NAPOLI 3, the incidence of Grade 3 or 4 neutropenia was similar among Asian patients [6 of 20 (30%)] compared to White patients [76 of 289 (26%)]. Neutropenic fever/neutropenic sepsis was reported in 5% of Asian patients (1 of 20) compared to 2.3% of White patients (7 of 306). In NAPOLI-1, Grade 3 and 4 neutropenia occurred in 20% of patients receiving ONIVYDE in combination with fluorouracil and leucovorin (ONIVYDE/FU/LV). Neutropenic sepsis occurred in 3% and fatal neutropenic sepsis in 0.8%. In NAPOLI-1, the incidence of Grade 3 or 4 neutropenia was higher among Asian patients [18 of 33 (55%)] compared to White patients [13 of 73 (18%)]. Neutropenic fever/neutropenic sepsis was reported in 6% of Asian patients compared to 1% of White patients.
Monitor complete blood cell counts on Days 1 and 8 of every cycle and more frequently if clinically indicated. Withhold ONIVYDE if the absolute neutrophil count (ANC) is below 1500/mm3 or if neutropenic fever occurs. Resume ONIVYDE when the ANC is 1500/mm3 or above. Reduce ONIVYDE dose for Grade 3-4 neutropenia or neutropenic fever following recovery in subsequent cycles.
Severe Diarrhea: In NAPOLI 3, Grade 3 and 4 diarrhea (early-onset [within 24 hours of chemotherapy] and late-onset [more than 24 hours following chemotherapy]) occurred in 20% receiving NALIRIFOX. In NAPOLI-1, Grade 3 or 4 diarrhea occurred in 13% receiving ONIVYDE/FU/LV. The incidence of Grade 3 or 4 late-onset diarrhea was 9% in patients receiving ONIVYDE/FU/LV. The incidence of Grade 3 or 4 early-onset diarrhea was 3% in patients receiving ONIVYDE/FU/LV.
To reduce the risk of severe diarrhea, patients should stop lactose-containing products, eat a low-fat diet, and maintain hydration during treatment with ONIVYDE. Withhold ONIVYDE for Grade 2-4 diarrhea. Local institutional guidelines should be followed for the treatment of diarrhea that does not improve within 48 hours and may include the addition of diphenoxylate hydrochloride plus atropine sulfate or octreotide. Following recovery to Grade 1 diarrhea, resume ONIVYDE at a reduced dose.
Interstitial Lung Disease (ILD): ONIVYDE can cause severe and fatal ILD. Postmarketing cases of severe and fatal ILD have been reported with ONIVYDE. Risk factors include pre-existing lung disease, use of pneumotoxic medicinal products, colony stimulating factors or having previously received radiation therapy. Patients with risk factors should be closely monitored for respiratory symptoms before and during ONIVYDE therapy. Withhold ONIVYDE in patients with new or progressive dyspnea, cough, and fever, pending diagnostic evaluation. Discontinue ONIVYDE in patients with a confirmed diagnosis of ILD.
Severe Hypersensitivity Reaction: Irinotecan, including ONIVYDE, can cause severe hypersensitivity reactions, including anaphylactic reactions. Permanently discontinue ONIVYDE in patients who experience a severe hypersensitivity reaction.
Embryo-Fetal Toxicity: Based on animal data with irinotecan HCl and the mechanism of action of ONIVYDE, ONIVYDE can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during and for 7 months after the last dose of ONIVYDE treatment.
ADVERSE REACTIONS FOR NALIRIFOX
- The most common adverse reactions (≥20%) of NALIRIFOX were diarrhea (72%), fatigue (62%), nausea (59%), vomiting (40%), decreased appetite (37%), abdominal pain (35%), mucosal inflammation (28%), constipation (25%), and weight decreased (22%).
- Permanent discontinuation of ONIVYDE due to an adverse reaction occurred in 17% of patients. Adverse reactions that resulted in permanent discontinuation of ONIVYDE in ≥1% of patients included neutropenia, thrombocytopenia, diarrhea, fatigue, infections, and cerebrovascular accident.
- Dosage reduction of ONIVYDE due to an adverse reaction occurred in 52% of patients. Adverse reactions that required dosage reduction in ≥1% of patients included anemia, decreased appetite, diarrhea, fatigue, febrile neutropenia, hypokalemia, liver function test abnormalities, nausea, mucosal inflammation, neutropenia, peripheral neuropathy, vomiting, thrombocytopenia, and weight decreased.
- Dosage interruptions of ONIVYDE due to an adverse reaction occurred in 1.9% of patients. Adverse reactions which required dosage interruption in ≥0.5% of patients included hypersensitivity and infusion-related reaction.
- The most common laboratory abnormalities (≥10% Grade 3 or 4) were decreased neutrophils (26%), decreased potassium (22%), decreased lymphocytes (11%), and decreased hemoglobin (10%).
ADVERSE REACTIONS FOR ONIVYDE/FU/LV
- The most common adverse reactions (≥20%) were diarrhea (59%), fatigue/asthenia (56%), vomiting (52%), nausea (51%), decreased appetite (44%), stomatitis (32%), and pyrexia (23%).
- Adverse reactions led to permanent discontinuation of ONIVYDE in 11% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions resulting in discontinuation of ONIVYDE were diarrhea, vomiting, and sepsis.
- Dose reductions of ONIVYDE for adverse reactions occurred in 33% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions requiring dose reductions were neutropenia, diarrhea, nausea, and anemia.
- ONIVYDE was withheld or delayed for adverse reactions in 62% of patients receiving ONIVYDE/FU/LV; the most frequent adverse reactions requiring interruption or delays were neutropenia, diarrhea, fatigue, vomiting, and thrombocytopenia.
- The most common severe laboratory abnormalities (≥10% Grade 3 or 4) were lymphopenia and neutropenia.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of ONIVYDE:
- Hypersensitivity (including anaphylactic reaction and angioedema).
DRUG INTERACTIONS
- Avoid the use of strong CYP3A4 inducers, if possible, and substitute non-enzyme inducing therapies ≥2 weeks prior to initiation of ONIVYDE.
- Avoid the use of strong CYP3A4 or UGT1A1 inhibitors, if possible, and discontinue strong CYP3A4 inhibitors ≥1 week prior to starting therapy.
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data with irinotecan HCl and the mechanism of action of ONIVYDE, ONIVYDE can cause fetal harm when administered to a pregnant woman. There are no available data in pregnant women. Advise pregnant women of the potential risk to a fetus.
- Lactation: Advise nursing women not to breastfeed during and for 1 month after the last dose of ONIVYDE.
To report SUSPECTED ADVERSE REACTIONS, contact Ipsen Biopharmaceuticals, Inc. at 1-855-463-5127 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including BOXED WARNING, for ONIVYDE at ONIVYDE.com/USPI.


